Call Us: (920) 233-1540
Breast Implant Removal
In a breast implant removal (“explant”) surgery, the surgeon removes the breast implant or revises the shape of the implants. Women choose to have their implants removed for reasons such as:
- Personal choice: After breast augmentation, women may feel their breast implants are the wrong shape or size. This is because pregnancy, weight loss, and weight gain can cause implants to move into the wrong position. Fortunately, it is a relatively easy and common procedure to have a surgeon remove the implants.
- Implant complications: Breast implant complications include implant rupture, folding or deflation (if the breast implant is filled with saline).
- Other complications: Capsular contracture, tension bands asymmetry, displacement, infection and leakage of silicone. The FDA is currently investigating the terms Breast Implant Illness (BII) and Breast Implant Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). As such, for some, an important step in recovering from both BIA-ALCL and BII is to remove the breast implant (explant) and the capsule tissue in a timely and proper manner.
Symptoms of Breast Implant Illness (BII) may reduce or clear after explant surgery. Ultimately, there is no guarantee of improvement because implants may or may not truly attribute to the cause of the illness. There are no rigid criteria to define BII, other than that the patient believes that the implants are causing her to be ill. If that is so, we of course do not try to convince our patients otherwise.
Is Removal Right for You?
Request a consultation to learn more about breast explant surgery at FVPS using our online form or contact us at (920) 233-1540. Depending on your circumstances, the non-refundable consultation fee can range from $150 to $500. However, insurance may cover some breast implant removal consultations.
Our surgeons have performed many explant surgeries for our patients from all over the country and can help you determine the best course of action.
Breast Implant Safety According to FDA
 The FDA is looking at the safety of breast implants. In July 2019 the FDA requested that Allergan recall specific types of textured implants. Nonetheless, the agency’s longstanding position is that implants are essentially safe.
The FDA is looking at the safety of breast implants. In July 2019 the FDA requested that Allergan recall specific types of textured implants. Nonetheless, the agency’s longstanding position is that implants are essentially safe.
Women should understand they can have complications during breast augmentation, like any surgery. These include scarring, pain, swelling and implant rupture. However, the FDA is investigating a recently confirmed link to a rare cancer and unconfirmed complaints of other health problems.
The U.S. does not track the total number of implants on the market, which makes it impossible for the FDA to determine how frequently cancer occurs. Estimates of the frequency of the disease range from 1 in 3,000 women to 1 in 30,000. We have not yet had a case of this disease in our office, even after testing several patients.
Allergan sent letters to patients, who had provided a valid address as part of device tracking, of the recalled products in August 2019. Patients who had elected to ‘opt-out’ of device tracking as well as those with textured saline breast implants and tissue expanders have access to the same information through the Allergan website.
For more information, please contact Allergan Medical Information at 1-800-678-1605, option #2 or email [email protected].
What Causes BIA-ALCL?
Most confirmed cases of the disease, known as BIA-ALCL, have involved a particular style of implants. These include those with a textured surface, designed to reduce scar tissue and slippage. The type of texturing used by Allergan is more commonly associated with this problem.
The disease is not breast cancer. Rather, it is a form of cancer that attacks the immune system and usually forms in the capsular tissue surrounding implants. Surgically removing the implants with the body’s self-created capsule can successfully treat the disease because it grows slowly.
While everyone agrees that the governments needs to conduct more research and studies, the FDA is not recommending removal of implants unless there are adverse symptoms.
It would appear that all BIA-ALCL patients present first with hyperinflation or a large fluid accumulation around the breast. We establish the diagnosis by sampling the fluid with ultrasound guidance in our office and testing the fluid.
- If there are no symptoms of BIA-ALCL, the FDA is not recommending removal of the implants. However, if the patient knows that she has TEXTURED Allergan implants, the patient should schedule an appointment with her surgeon for more guidance. This is not an urgent appointment unless there are symptoms of the illness. Allergan has issued a Biocell Replacement Warranty in response to the recall.
- The patient should call our office to check if unsure of her type of implant. Please allow the office up to 5 business days to retrieve this information.
- If the patient had her implants placed elsewhere, we need the medical records from the original surgeon. Upon record review, we will determine if we can see the patient.
Terminology for Breast Implant Removal (Explantation)
It is important to understand the terms when we are talking about this subject.
Implant
The actual silicone or saline breast implant.
Explant
The act of removing a breast implant.
Capsule
The tissue envelope the body forms around the implant. The capsule did not exist before the implant. The body forms a capsule around any foreign object in the tissue. Because the body does not know if the foreign object is good or bad, it walls it off from the body. The body can actually absorb the capsule if the implant is removed. An ultrasound can confirm this.
Capsular Contracture
The thickening, tightening, or calcification of the capsule. When you have a “hard implant” the implant is not hard. It refers to the capsule. A subclinical infection (Biofilm) around the implant is thought to cause capsular contracture.
Capsulectomy
The act of removing the capsule around the implant. Surgeons usually perform a capsulectomy for either capsular contracture or permanent removal of the implant. This does not necessarily mean all capsule tissue is removed, as a capsulectomy can be partial or complete/total. TOTAL Capsulectomy is removal of the entire capsule.
En Bloc Removal
The surgeon removes the capsule and the implant in one piece with the implant still within the wall of the capsule. During this type of surgery, the surgeon needs to minimize any silicone leakage or other problems. As such en bloc removal makes this a more complicated procedure than placing implants.
It requires a larger incision, which means a larger scar. Patients who had an axillary or periareolar incision, cannot have the procedure done through those incisions.
Unfortunately, the surgeon may not know if an en bloc procedure is truly possible until the actual surgery occurs. Sometimes the surgeon needs to disturb a portion of the capsule to avoid significant damage to the muscle, rib or lung.
Actual En Bloc Case
If the scar tissue capsule is very thin or tightly attached to the chest wall, the surgeon may attempt en bloc removal. However, there is always a possibility of breaking into the capsule, especially along the ribs.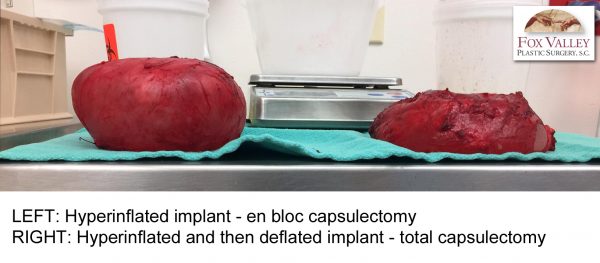 In the above case, Dr. Janssen successfully removed the entire capsule intact (en bloc capsulectomy) for the left hyperinflated implant. On the right implant, he attempted en bloc unsuccessfully, as indicate by the hole in the capsule. He removed the posterior wall along with some muscle fibers with the complete capsule in the 2-hour dissection. Ultimately, this patient did not have BIA-ALCL.
In the above case, Dr. Janssen successfully removed the entire capsule intact (en bloc capsulectomy) for the left hyperinflated implant. On the right implant, he attempted en bloc unsuccessfully, as indicate by the hole in the capsule. He removed the posterior wall along with some muscle fibers with the complete capsule in the 2-hour dissection. Ultimately, this patient did not have BIA-ALCL.
The dual purpose of textured implants was to stabilize the implant in the breast pocket and to reduce capsular contracture. However, they are the most confirmed with causing BIA-ALCL, a cancer of the immune system that develops in the capsules and creates fluid near the implant.
En bloc removal of textured implants is the best way to keep the cancerous cells secured in the capsule as a sac until they are explanted out of the body, as a whole. Patients, who have textured implants removed, may request pathology to do the CD30 test for BIA-ALCL.
In 30 years of practice, we have never seen a case of this disease. The En Bloc technique is best used when an implant is ruptured. Then we try to remove the capsule, implant, and tissue that contains silicone in one piece.
Total Capsulectomy
Some refer en bloc removal as a total capsulectomy, but they are not exactly the same. One does not always perform a total capsulectomy using the specific en bloc technique. A total capsulectomy is the removal of the breast implants and all the scar tissue, or capsule, that forms around a breast implant. However, during a total capsulectomy, one may not necessarily remove the implant while still inside the intact scar capsule (en bloc method).
Many surgeons use the term total capsulectomy to mean that they will cut the scar tissue capsule, remove the implants first, and then go back and remove all the scar tissue capsule. One may perform a complete capsulectomy with the implant out through a smaller incision than a capsulectomy with the implant in. The right operation depends on the patient’s needs and desires.
If you want an en bloc capsulectomy, make this clear to the doctor because it is not normally performed unless needed or asked.
The Aesthetic Surgery Journal published a study in December 2021 with the following conclusion:
“The type of capsulectomy; intact total, total, or partial all showed similar symptom improvement with no statistical difference in the reduction of symptoms based on the type of capsulectomy.”
To read the study, please click here.


Oshkosh, WI (920) 233-1540